OCEAN ACIDIFICATION: THE OTHER CARBON DIOXIDE PROBLEM
Ocean acidification is the term given to the chemical changes in the ocean as a result of carbon dioxide emissions.
Approximate 25% of the steadily increasing amount of atmospheric CO2 emission is absorbed by the oceans.
The oceans are an enormous and play a key role in helping regulate the amount of CO2 in the atmosphere
OCEANS AND C02
The oceans are an enormous store of carbon far more than on land or in the atmosphere and hence play a key role in the global carbon cycle, especially in helping regulate the amount of CO2 in the atmosphere. The oceans are important because they have taken up around 28-34% of the CO2 produced by humankind.
For some extend oceans are reducing the extent of greenhouse warming and climate change
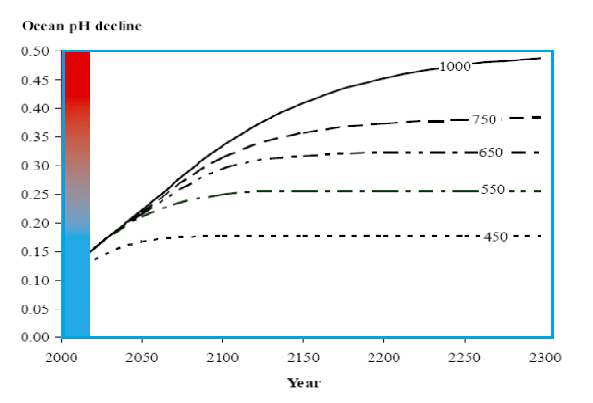
Trajectories for surface ocean pH decrease calculated for different atmospheric CO2 concentration models profiles leading to stabilization from 450-100 ppm. These were calculated from the model predictions by Caldeira & Wickett .
UNPRECEDENTED RATE OF ACIDIFICATION
Fundamental changes in seawater chemistry are occurring throughout the world's oceans. Of great concern is the approximately 30% decrease in ocean pH and 16% decrease in carbonate ion concentration between pre-industrial days and today. The measured impact of CO2 emission on the ocean carbonate chemistry predicts with very high certainty that ocean acidification will continue as it is directly related to the CO2 emissions in to the atmosphere.
Gathered evidence from experiments and observations over the last decades are raising concern that further ocean acidification may be a threat to many marine organisms. This is mainly due to the rapid rate at which ocean carbonate chemistry is changing.
This exert pressure on marine organisms to respond.
Responses can come in many forms :
- Marine organisms may migrate to alternate habitats,
- Others may be able to acclimatize to changes in their environment.
- Those that cannot may not survive these rapid changes to ocean chemistry.
These different scenarios will have wider implications on food webs and ecosystem function.
It is largely unknown what the what the scale and direction of impacts due to ocean acidification will be, such as:
- An altered biochemical cycling of carbon,
- Effect on ocean nutrients
An interpretation on bases on geologic analogues in the Earth’s past is not possible. Simply because there is no exact geological analogue because the present rate of acidification today is faster than at any time in the last 65 million years. Furthermore the oceans chemistry is different today than during large parts of Earth’s history
FOOD WEB AND ECOSYSTEM
When CO2 is absorbed by seawater, chemical changes occur that reduce seawater pH and the concentration
of carbonate ions. The pH (the measure of acidity) of ocean surface waters has already decreased by about
0.1 units, from an average of about 8.2 to 8.1 since the beginning of the industrial revolution. The acidification is logarithmic, meaning : 0.1 Ph unit is an acidification of about 26% percent.
Carbonate ions are a basic building block of skeletons and shells for a large number of marine organisms, including corals, shellfish, and marine plankton. Some of these smaller calcifying plankton are important food sources for higher marine organisms.
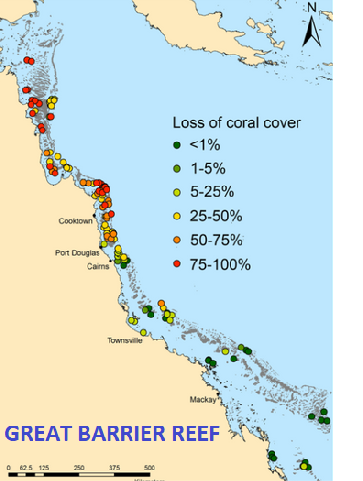
Coral reefs are home to at least a quarter of the entire biological diversity of the oceans. Coral reefs serve as important habitat to as many as 1 to 3 million species, including more than 25% of all marine fish species.
Species feed, reproduce, shelter and survive in this vast 3-dimensional framework offered by coral reefs.
The combined pressures of increasing acidity and global warming makes that coral reefs are becoming nothing more than eroded rock platforms.
In coming decades ocean acidification could have deleterious impacts for some species. On the other hand some species may even prosper. However, it is not known in what way these biochemical changes and interactions will alter the ecosystem as a whole.
It is not sure if the ecosystem will sustain in servicing the present food webs and ecosystems















 CO2 remains in the atmosphere longer than the other major heat-
CO2 remains in the atmosphere longer than the other major heat-